As standard of care, all critically ill patients require routine neurologic examination, including pupillary assessment. Patients with primary or secondary neurological insult are at risk for development of hydrocephalus, cerebral edema, elevated intracranial pressure (ICP), and brain herniation. Accurate serial neurological assessment is imperative to 1) rapidly detect changes that may indicate neurologic deterioration, 2) guide patient management, 3) determine the effect of therapeutic interventions and 4) inform prognosis.
Assessment of pupil size and the pupillary light reflex is a critical component of the neurological exam for patients in various settings (26, 50, 55) such as the Emergency Department (ED), Intensive Care Unit (ICU), Operating Room (OR), Post Anesthesia Care Unit (PACU), diagnostic areas such as Interventional Radiology (IR), and Progressive Care Units (PCU). Pupillary changes correlate with neurological worsening and yet are often undetectable using traditional manual assessment methods. Manual pupillary assessment, using a flashlight or penlight, is prone to many sources of inaccuracy and is characterized by subjectivity and low interrater reliability. (1, 14, 26, 39, 46, 48, 56, 60)
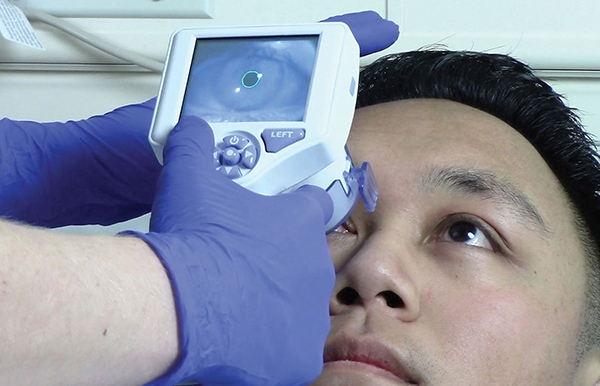
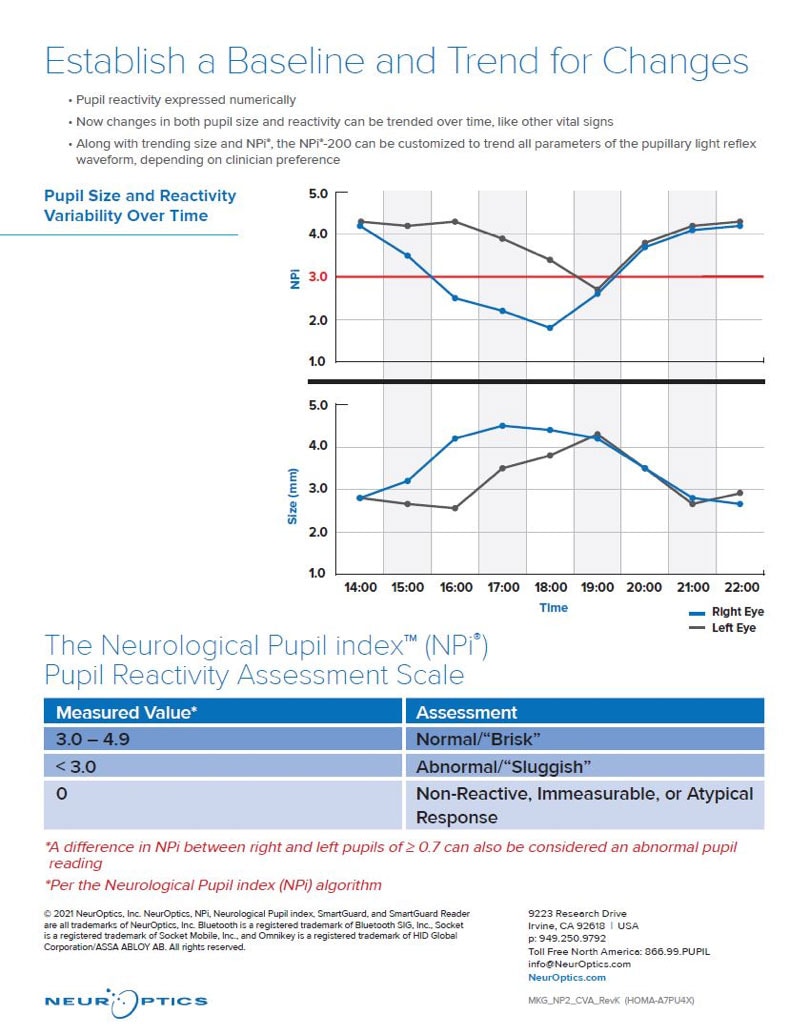
The NeurOptics NPi®-200 Pupillometer is a handheld, automated device that provides an accurate, reliable, and objective measurement of pupillary size, symmetry, and reactivity through measurement of the entire pupillary light reflex (PLR). The Neurological Pupil index (NPi®), calculated by the Pupillometer, reflects a comparison of all measured variables in the PLR to known normal observations. On the numeric NPi® scale, an NPi® from 3.0 to 4.9 is considered normal, while an NPi® less than 3.0 is considered abnormal. (9, 14, 19, 43, 46, 48, 60,66)
The following findings have been objectively demonstrated when quantitative pupillometry is utilized:
i. Improvement in inter-rater reliability of pupillary metrics (1, 46, 48)
ii. Accuracy of measurement in common critical care scenarios (sedation and pharmacologic paralysis) (23, 30, 31, 42, 45, 58)
iii. Early indication of neurological worsening (cerebral edema, elevation in ICP, delayed cerebral ischemia, identification of herniation syndromes) (1, 5, 14, 19, 27, 29, 30, 39, 49, 51, 52)
iv. Monitoring response to therapeutic interventions (hyperosmolar therapy) (27, 29, 49)
v. Prognostic value after traumatic brain injury, cardiac arrest and ECMO (27, 42, 45, 57)
Pupil Examination Is a Standard of Care and Important to Patient Outcome

The clinical neurological exam is an essential component in the assessment and care of patients with a wide variety of illnesses and injuries.(28, 34, 46) For centuries, clinicians have assessed the pupils of patients with impaired consciousness. Today, clinicians routinely evaluate pupils as part of the neurological exam and monitoring of all critically ill patients, including those with primary neurological injury as well as those at risk of secondary neurologic insults.(4, 26, 34, 41, 46, 47, 50, 55, 63) Pupillary examination involves assessment of the functional status of two cranial nerves (CN) – the optic nerve (CN II) and the oculomotor nerve (CN III). Cranial nerve dysfunction may signal increased ICP and/or an increased risk of brain herniation. (2, 46)
Pupillary information is relied on to guide decisions regarding patient triage and clinical intervention. Depending on pupillary status, neurosurgeons triage patients into either conservative therapy or surgical evacuation of mass lesions.(46, 50, 56) Patients who undergo prompt intervention such as surgery or hyperosmolar therapy after a new finding of pupil abnormality have a better chance of recovery.(20)
Abnormalities of pupillary response are associated with neurological deterioration and correlate with poor neurological outcomes.(4, 14, 20, 55, 62) Along with other clinical information such as age, mechanism of injury and Glasgow Coma Score (GCS), the pupillary light reflex is a useful prognostic indicator of survival, functional recovery and long-term outcome.(4, 20, 63)
Manual Pupil Assessment Is Highly Subjective and Inaccurate

Traditionally, pupil assessment has been performed in a subjective manner, using a penlight or flashlight to manually evaluate pupil reactivity and a pupil gauge to estimate pupil size. Common terminology employed to describe the pupillary light reflex and pupil size includes “fixed” or “dilated” as well as “brisk”, “sluggish” or “non-reactive” pupils. These subjective terms are applied without a standardized clinical definition and yield a pronounced level of inter-examiner variability and error. (17, 41, 48)
Manual pupillary assessment is subject to compounded sources of inaccuracies and inconsistencies and can result in as much as 39% inter-examiner variability. (4, 17, 26, 28, 40, 41, 46, 48) A variety of factors affect the reliability of manual assessment and increase inter-examiner disagreement. These factors may include pinpoint pupils, darkly pigmented irises, examiner skill level and visual acuity, and the strength and orientation of the light stimulus with respect to the patient’s eye.(2, 36) A 2016 study revealed that critical care and neurosurgical nurses consistently underestimated pupil size, were unable to identify anisocoria, and incorrectly assessed pupil reactivity. (28, 43)
The NPi®-200 Pupillometer Is Reliable and Eliminates Subjectivity

The NeurOptics NPi®-200 Pupillometer (NeurOptics, Inc., Irvine, CA) is a Class 1 device that is 510k Exempt by the US FDA and CE-marked for the European Economic Area for use in both adults and children. This noninvasive, handheld optical scanner provides reliable and objective measurements of pupillary size, symmetry, and reactivity. The device stimulates pupil constriction with a gentle flash of light as the infrared camera captures 90 images in a 3 second period, measuring 8 different parameters that comprise the entire pupillary light reflex. A disposable SmartGuard®, programmed with a unique patient identifier, records and stores 168 paired pupil assessments for trending and review throughout the patient’s admission. As part of the NPi®-200 Pupillometer System, the optional barcode scanner and SmartGuard® Reader allow automated patient identification entry and upload of data to the electronic medical record.
Quantifying pupil reactivity on a numeric scale (from 0 to 4.9), the Neurological Pupil index (NPi®) allows rigorous interpretation and classification of the pupil response. The Pupillometer and the NPi® Pupil Reactivity Assessment Scale provide objectivity in measurement by comparing the patient’s pupillary light reflex to normative data in the NPi® model. By automatically deriving whether the pupillary light reflex falls within the normal range (> 3.0 or “brisk”), the abnormal range (< 3.0 or “sluggish”) or is “atypical”, “immeasurable” or “non-reactive” (0), the NPi®-200 Pupillometer provides a reliable and accurate way to quantify and trend the pupillary light response, offering increased confidence in the neurological assessment. (4, 7, 14, 17, 21, 23, 41, 46, 48)
Monitoring, Treatment and Prognostic Value in Critical Care
Recognized as an important tool in the ICU, automated pupillometry is being adopted in hospitals worldwide and becoming accepted as a standard of care for pupillary assessment in patients with a variety of critical illnesses.(2, 55, 69)
Neurocritical Care
Use of the NPi-200 Pupillometer has proven to be an objective means of assessing and trending pupillary reactivity across a broad spectrum of neurological diseases including traumatic brain injury (TBI), ischemic and hemorrhagic stroke, cerebral edema, and herniation syndromes. (5, 11, 15, 23, 32, 44) Automated Pupillometer technology demonstrates emerging value as a prognostic tool, with predictive properties that could allow practitioners to anticipate neurological injury as well as recovery. (6, 32, 44, 55)
TBI
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide, accounting for around 30% of all injury-related deaths. (27) Accurate neurological and pupillary assessment are critical for detection of neurological deterioration. A study published in May 2019 investigated the relationship between non-invasive measures of NPi and invasive ICP monitoring in TBI patients at risk for intracranial hypertension. This important study concluded that abnormal NPi was a marker of severity of the increased ICP, a more complicated course and a worse outcome at 6 months. (27) A 2020 study of ED patients presenting with blunt TBI showed that higher NPi’s were associated with better outcome and lower modified Rankin Scores, while NPi’s <3.0 were associated with a more severe brain injury requiring emergent surgical intervention (placement of an ICP monitor, ventricular drain, or craniotomy). (19)
Ischemic and Hemorrhagic Stroke
Several recent studies have examined the relationship between NPi and neurological worsening after stroke. Findings from a 2018 study of patients experiencing vasospasm after subarachnoid hemorrhage revealed that NPi <3.0 was strongly associated with the onset of delayed cerebral ischemia and that NPi often decreased >8 hours prior to clinical deterioration. (5) A study of patients with large hemispheric stroke showed that neurological worsening often occurred following a sudden drop in NPi and was associated with all NPi values <2.8. (30) Using data from a national registry, a significant statistical relationship was found between NPi and midline shift in patients with ischemic stroke and intracerebral hemorrhage (ICH). (51) Shift of midline structures, hematoma volume and other CT scan indicators of hemorrhage severity have also been found to correlate with NPi in ICH patients. (38)
Cerebral Edema and Increased ICP
Cerebral edema and increased ICP are known potential complications of many disorders and can result in neurological deterioration and herniation syndromes. Numerous studies have shown the relationship between NPi and cerebral edema, ICP, and neurological deterioration. (1, 14, 27, 29, 44, 49, 52) Beginning in 2011, studies have increasingly demonstrated the relationship between lower values of NPi (<3.0) and higher values of ICP in patients with a variety of neurological diagnoses. (1, 14, 39, 40)
Status Epilepticus (SE)
The term status epilepticus is applied to continuous seizure activity characterized by impairment of consciousness or behavior and may be classified as convulsive or non-convulsive. Diagnosed by electroencephalogram (EEG), SE is common in critically ill patients and is a neurological emergency that can lead to brain damage and death.(9,26) A recent study found that NPi was significantly reduced, and the difference between left and right NPi was significantly higher in patients diagnosed with non-convulsive status epilepticus (NCSE).(23) All patients diagnosed with NCSE in this study were correctly identified by pupillometry. This study is the first to suggest pupillometry as a helpful diagnostic tool in the assessment of NCSE.
Other Critical Illnesses
Automated pupillometry is also proving beneficial to patients without a primary neurologic diagnosis – those with potential for neurologic complications from such conditions as cardiac arrest, liver failure, acute respiratory distress syndrome (ARDS), and sepsis; and in procedures such as mechanical ventilation and extracorporeal membrane oxygenation (ECMO).
Cardiac Arrest
Sudden cardiac arrest continues to be a major public health crisis, with over 350,000 people in the U.S. (all ages) suffering out-of-hospital cardiac arrest each year. Despite advances in layperson CPR and prehospital care, survival rates languish at approximately 10%. Care of the patient after return of spontaneous circulation (ROSC) requires close attention to oxygenation and blood pressure, targeted temperature management (TTM), and multimodal neuroprognostication. (3)
Several studies have highlighted the prognostic value of pupillometry in cardiac arrest patients. In earlier studies, the presence of the pupillary light reflexes obtained in serial measurements during resuscitation was associated with early survival and a favorable neurological status in the recovery period. (8, 12) More recent studies have shown that quantitative NPi has excellent ability to predict an unfavorable outcome after cardiac arrest, with significantly higher specificity than standard manual pupillary examination. (43) A multicenter international trial in 2018 showed that NPi was 100% specific to predict an unfavorable outcome at 3 months in comatose patients treated with TTM after cardiac arrest. (45) Shortly thereafter, a U.S. single center study confirmed the ability of NPi, obtained within 6 hours of ROSC, to predict poor clinical outcome, independent of pupil size. (57)
As a result of these studies and others, pupillometry and the Neurological Pupil index (NPi) are now included in the updated 2020 American Heart Association (AHA) Guidelines for Cardiopulmonary Resuscitation (CPR) and Emergency Cardiovascular Care (ECC) as an objective measurement supporting brain injury prognosis following cardiac arrest. (3) Expansion of the AHA guidelines to include this technology reflects the growing adoption and validation of automated pupillometry and NPi.
ECMO
Despite its benefits, venoarterial extracorporeal membrane oxygenation (VA-ECMO) after refractory cardiogenic shock or cardiac arrest carries significant risk of morbidity and mortality. Early outcome prediction is crucial but challenging in this setting. A 2020 study of patients on VA-ECMO found that NPi had excellent ability to predict poor outcome from day 1, with an NPi <3, at any time between 24 and 72 hours of initiation being 100% specific for 90-day mortality. (42)
Emergency Department (ED)
Triage
The ability to rapidly triage, diagnose and treat (or transfer) patients is imperative in the setting of emergency medicine. By analyzing the relationship between NPi and surgical intervention (placement of an ICP monitor or craniotomy), a 2020 pilot study conducted in the ED of a busy Level I trauma center, demonstrated the value of NPi in the triage and treatment of patients with blunt TBI. Researchers found that NPi <3 was predictive of the need for emergent surgical intervention, while NPi was normal (>3) in all patients who did not require intervention. (19) The authors recognized the potential application of these findings to first responders (both civilian and military) to assist in prioritizing the transfer of TBI patients to appropriate centers of care.
Altered Mental Status (AMS)
The pupillometer and NPi have practical applications for patients presenting to the ED with altered mental status. Defined as a change in cognitive function or level of consciousness, AMS is a common reason for ED visits, hospitalization, and neurologic consultation. Up to 10% of ED patients present with AMS, with higher percentages in the elderly population. (16, 24, 61, 68) Prompt evaluation and treatment are essential to reduce the morbidity and mortality associated with AMS.(61, 68)
With wide ranging etiologies (including trauma, intoxication, autoimmune and metabolic disorders, stroke, seizure and many others), the World Health Organization recommends an organized, systematic method of evaluation using the A (Airway), B (Breathing), C (Circulation), D (Disability) and E (Exposure) approach. (67) The D (Disability) assessment includes pupillary size, symmetry and reactivity, and suggests possible causes of AMS based upon pupil findings. The NPi-200 Pupillometer is instrumental in improving the accuracy and reliability of this critical assessment.
Teleneurology
Teleneurology is an evolving branch of telemedicine using various methods of connectivity, such as telephone or internet, to provide remote consultation for patients suffering from neurological issues such as AMS, stroke, or TBI. (22) Often linking outlying facilities having fewer services to more specialized full-service facilities, teleneurology allows clinicians to determine if a patient’s condition is best diagnosed and treated at the presenting facility or warrants transfer to a higher level of care. Goals of teleneurology include improved quality of care, increased access to specialists, shorter time to treatment, reduction of inappropriate transfers, increased patient and family satisfaction, shorter length of stay, improved cost and resource efficiencies, and improved patient outcome. (22, 25, 65)
Regardless of whether a patient remains at the presenting hospital or is transferred to a higher level of care, studies support the use of Pupillometry to obtain accurate, reliable, and objective pupil assessment data which is critical to decisions regarding diagnosis, treatment, and transfer. Preserving the continuity of use of pupillometry from the presenting facility to the receiving facility ensures an accurate baseline assessment and ongoing objective, trendable data on which to base treatment decisions and prognosis. For all the same reasons that the Pupillometer is used in top neurology and neurosurgery centers, it should be a standard part of neuro assessment and monitoring for all facilities within the teleneurology network.
Summary and Future Focus
The creation of databases and ongoing research are providing a greater understanding of expected distributions for normative pupillometry values upon which future studies and clinical practice can build.(33, 47) Burgeoning areas of research and publication include the use of automated pupillometry in pediatric populations, (10, 13, 18, 53) application to military medicine, (19,52) and in evaluation of pharmacodynamic activity of drugs, such as opioids and anesthetic agents. (37, 54, 58)
In summary, pupil evaluation is a critical component in the assessment and management of critically ill patients with direct neuronal injury or potential neurologic sequela. Manual pupil measurements are characterized by high inter-examiner variability and error. As an optimized method of neurological assessment, the NPi-200 Pupillometer has the potential to improve detection of conditions leading to neurologic deterioration, serving as an early indicator of worsening intracranial pathology and clinical deterioration. (5, 43, 51, 52) Use of the Pupillometer and the NPi Pupil Reactivity Assessment Scale is proving beneficial in guiding treatment decisions for early therapeutic intervention, in evaluating the effects of clinical interventions, and in providing important prognostic information. (6, 28, 32, 35, 36, 43, 49, 56)
Quality Improvement
Quality measures help quantify healthcare processes, outcomes, patient perceptions, and organizational structure. They are associated with the ability to provide high-quality care and relate to one or more quality goals for health care. These goals include effective, safe, efficient, patient-centered, equitable, and timely care. Ongoing quality improvement is a cornerstone for all hospitals and automated pupillometry is a natural fit as hospital quality systems continue to evolve.
Improved Quality of Neurological Exam
Assessment of pupil size and reactivity is a fundamental aspect of the neurological examination. However, manual pupil assessments (using a penlight or flashlight) are subjective and fraught with a high degree of inter-examiner variability. In a 2015 study from The University of Texas Southwestern Medical Center, two examiners under identical conditions evaluated the pupils of consented patients. Over 2300 paired assessments were performed, followed by assessment with the pupillometer. The study showed only moderate inter-rater reliability between practitioners for pupil size, shape and reactivity. Additionally, only 33% of pupils scored as non-reactive by practitioners were scored as non-reactive by pupillometry. (48) This study and others support the use of automated pupillometry to improve the reliability and objectivity of pupillary assessment.
A quality improvement project, published by The University of Pittsburgh Medical Center, looked at adoption of the Pupillometer for routine care in a Neurotrauma Intensive Care Unit. This center reported that the Pupillometer led to increased confidence in the neurological examination, enhanced clinical decision making, and added value to patient care. (4)
Automated pupillometry provides completely accurate, reliable and objective pupil data, independent of examiner, resulting in quality improvement for this important component of the neurological examination.
Adherence to Hospital Protocols/Reduction in Manual Entry Errors
Standard hospital protocols and guidelines for neurologically injured patients call for pupil assessments at regularly scheduled intervals. A typical nursing day with a critically ill patient requires coordination of care, diagnostic testing, frequent assessment and monitoring, medication administration, supporting the patient’s family, and many other complex and time-sensitive tasks. To save time and reduce error often associated with manual data entry, patient data can be automatically uploaded to most electronic medical record systems using the optional SmartGuard Reader. This provides hospitals with another means to monitor compliance with protocols, guidelines and quality initiatives. In addition, the date and time stamp allow data review and trending from the time of admission throughout the patient’s hospital course.
Staffing: Cost and Time Savings
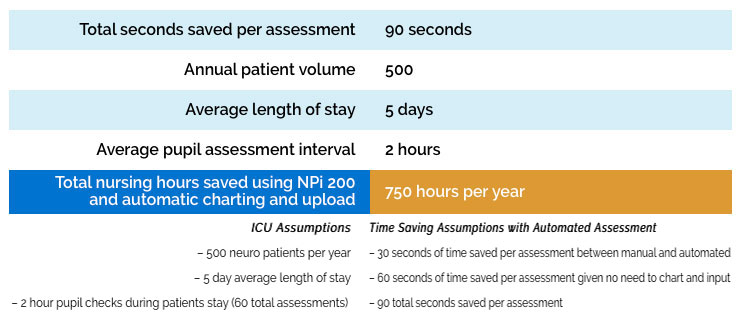
Automated pupil assessments have been shown to decrease nursing workload associated with time spent per assessment every 1-2 hours. As nursing leadership looks to improve efficiency in workflow and staffing, Pupillometers at each bedside can assist in that effort. See the example below in which utilization of pupillometry was shown to decrease nursing workload and was associated with time savings compared to manual assessments. (55)
Manual Pupil Assessment Technique
Manual pupil assessments (penlight) are subjective and fraught with a high degree of inter-examiner variability. It is well documented that automated pupillary assessment solves these problems, but manual pupil assessment can also take more time than automated pupillometry and little has been discussed about this.
A paired assessment with the pupillometer takes a clinician about 15 seconds. Manual pupil assessment can be confounded by several issues: Visual acuity of the examiner, dark eyes or small pupils of the patient, ambient light conditions and the subjectivity and skill/experience level of the examiner. As a result, in some patients, a clinician will often pass the penlight multiple times to try and confirm what he/she sees and a manual assessment can take even more time. In other cases, if the clinician is not sure, or if there is a suspected pupil change, another opinion might be sought. This involves an additional person and several minutes more of time. As a single measurement, these differences might not seem meaningful but given the frequency of pupil exams, these differences are significant over time.
Charting and Inputting Data
Some clinicians will complete the exam and immediately record the pupil measurement data into the terminal, while others will complete the pupil exam, continue with necessary patient care, and then later go to the terminal, log in, input the data and log out. Nurses at a high volume neuro ICU were recently timed and, on average, these charting and data entry steps took about one minute. Automatic data upload will eliminate the need to manually chart and input data.
Annual Cost Savings Example
Building an Effective Pupillometry Program
To achieve the benefits previously identified, successful adoption of pupillometry relies upon collaboration of a multidisciplinary team including nurses, providers, nursing leadership, Biomedical Engineering, Information Technology, and Supply Chain Management. Additionally, there are three key components to building an effective pupillometry program – eliminating barriers, establishing protocols and education.
Eliminating Barriers
Anticipating and eliminating barriers promotes successful adoption of new technology. Ensuring an appropriate number of and easy access to Pupillometers based on unit size/layout is essential for optimizing nursing workflow and routine. Many hospitals dedicate one pupillometer to each patient room, or one to every other room. A recent study showed that allocation of one pupillometer per patient room significantly improved compliance with pupillary assessment among nurses in the Neuro ICU.(59) A standardized process for ordering and stocking SmartGuards is equally important to ensure uninterrupted availability for patient care.
Another consideration is planning for documentation, including which values will be documented (typically pupil size and NPi) and in what format – paper flowsheet, fields added for manual EMR entry, or automatic upload. Examining their five-year experience with pupillometry and the recent ability to interface with the EMR, nurses across 6 different patient care units at University of California Irvine Health found that directly uploading pupillometer data to the EMR improved nursing workflow and improved documentation of pupillary findings and changes. (64) More information on EMR integration can be found at https://neuroptics.com/emr-integration/
Establishing Protocols
Much of the care provided to critically ill patients is driven by protocols based on evidence and best-practice guidelines. To standardize use and provide the most benefit to patients and clinicians, it is important to establish unit protocols and guidelines for use of the NPi-200 Pupillometer. Essential components of a comprehensive protocol include 1) clear indications for use, including both primary neurological injuries as well as secondary indications (CA, ARDS, AMS etc); 2) a focus on baseline assessment at the time of admission along with ongoing trending of values; 3) procedural steps for use; and 4) guidelines for documentation and reporting abnormal values. Sample protocols are available from your NeurOptics representative for use when developing protocols or guidelines in your institution.
Education
Nursing and provider education is essential to success of Pupillometer integration into clinical practice. NeurOptics stands ready to support education with local sales representatives and a network of nurse Clinical Specialists. Together with clinical educators/managers and key personnel, NeurOptics works to develop a comprehensive day/night/weekend education plan according to institutional requirements. While on-site education is preferred, virtual education in a variety of formats can also be provided.
Initial education, at the time of pupillometer installation/rollout, focuses on operation of the NPi-200 Pupillometer and conceptual understanding of its clinical use and benefits. Follow up education, scheduled four to six weeks later and periodically as needed, aims to reinforce operational skills and integration of pupillometry into routine clinical practice. Our education team is also available to support annual skills/competency days, Grand Rounds and other formal presentation sessions.
NeurOptics provides and promotes many educational resources on the website (www.NeurOptics.com), including product information, instructional videos, webinar recordings, and clinical publications.
AACN and AANN
AACN and AANN
The American Association of Critical-Care Nurses (AACN) Procedure Manual for High Acuity, Progressive and Critical Care features a new section on pupillometry, noting that the pupillary examination is a key assessment performed during neurological examinations and guiding nurses on the use of automated pupillometers.
The new edition of the American Association of Neuroscience Nurses (AANN) Core Curriculum for Neuroscience Nursing includes a new section on automated pupillary assessment, which states that use of a pupillometer removes measurement subjectivity and provides a way to track and trend pupillary reactivity in a consistent, objective, and quantifiable way.


Clinical Reference Texts Including Pupillometry and NPi

3a. American Heart Association. Highlights of the 2020 American Heart Association Guidelines for CPR and ECC; 2020 (3)
3b. Wiegand DJL-M, American Association of Critical-Care Nurses. AACN procedure manual for high-acuity, progressive, and critical care. Seventh edition. ed. Elsevier;2017:xxiv, pp880-884.(66)
3c. Bader M, Littlejohns L, Olson D, eds. AANN Core Curriculum for Neuroscience Nursing. 6th ed. American Association of Neuroscience Nurses; 2016 135-167:chap 7.(9)
3d. Hickey JV, Strayer A. The clinical practice of neurological and neurosurgical nursing. Eighth edition. ed. Wolters Kluwer; 2020:xvi, pp168-170. (26)
Appendix
Fig. 1 The NPi-200 Pupillometer System with optional Barcode Scanner and SmartGuard Reader

Fig. 2 The Neurological Pupil index (NPi) Pupil Reactivity Assessment Scale
Bibliography and Links to Abstracts
Clinical Publications Cited
9. Berlin T, Cecil S, Olin K, Puccio A. Technology. In: Bader M, Littlejohns L, Olson D, eds. AANN Core Curriculum for Neuroscience Nursing. 6th ed. American Association of Neuroscience Nurses; 2016 135-167:chap 7.
11. Bower MM, Sweidan AJ, Xu JC, Stern-Nezer S, Yu W, Groysman LI. Quantitative pupillometry in the intensive care unit. J Intensive Care Med. Oct2019:885066619881124. doi:10.1177/0885066619881124
16. Douglas VC, Josephson SA. Altered mental status. Continuum (Minneap Minn). Oct2011;17(5 Neurologic Consultation in the Hospital):967-83.doi:10.1212/01.CON.0000407055.17661.33
19. El Ahmadieh TY, Bedros NM, Stutzman SE, et al. Automated pupillometry as a triage and assessment tool in patients with traumatic brain injury. World Neurosurg. Oct 2020; doi:10.1016/j.wneu.2020.09.152
22. Freeman WD, Barrett KM, Vatz KA, Demaerschalk BM. Future neurohospitalist: teleneurohospitalist. Neurohospitalist. Oct 2012;2(4):132-43.doi:10.1177/1941874412450714
24. Han JH, Wilber ST. Altered mental status in older patients in the emergency department. Clin Geriatr Med. Feb 2013;29(1):101-36. doi:10.1016/j.cger.2012.09.005
25. Hatcher-Martin JM, Adams JL, Anderson ER, et al. Telemedicine in neurology: Telemedicine Work Group of the American Academy of Neurology update. Neurology. 012020;94(1):30-38. doi:10.1212/WNL.0000000000008708
26. Hickey JV, Strayer A. The clinical practice of neurological and neurosurgical nursing. Eighth edition. ed. Wolters Kluwer; 2020:xvi, pp168-170.
38. Mazhar K, Olson DM, Atem FD, et al. Supratentorial intracerebral hemorrhage volume and other CT variables predict the neurological pupil index. Clin Neurol Neurosurg. Jan 2021;200:106410. doi:10.1016/j.clineuro.2020.106410
43. Morelli P, Oddo M, Ben-Hamouda N. Role of automated pupillometry in critically ill patients. Minerva Anestesiol. Sep 2019;85(9):995-1002. doi:10.23736/S0375-9393.19.13437-2
44. Natzeder S, Mack DJ, Maissen G, Strässle C, Keller E, Muroi C. Portable infrared pupillometer in patients with subarachnoid hemorrhage: Prognostic value and circadian rhythm of the Neurological Pupil index (NPi). J Neurosurg Anesthesiol. Oct2019;31(4):428-433. doi:10.1097/ANA.0000000000000553
50. Ong C, Hutch M, Smirnakis S. The effect of ambient light conditions on quantitative pupillometry. Neurocrit Care. 04 2019;30(2):316-321. doi:10.1007/s12028-018-0607-8
55. Phillips SS, Mueller CM, Nogueira RG, Khalifa YM. A systematic review assessing the current state of automated pupillometry in the NeuroICU. Neurocrit Care. 082019;31(1):142-161. doi:10.1007/s12028-018-0645-2
56. Rasulo FA, Togni T, Romagnoli S. Essential noninvasive multimodality neuromonitoring for the critically ill patient. Crit Care. Mar 2020;24(1):100. doi:10.1186/s13054-020-2781-2
61. Smith AT, Han JH. Altered mental status in the emergency department. SeminNeurol. 02 2019;39(1):5-19. doi:10.1055/s-0038-1677035
65. Wechsler LR. The teleneurology revolution. Ann Neurol. Oct 2020;88(4):656-657. doi:10.1002/ana.25849
66. Wiegand DJL-M, American Association of Critical-Care Nurses. AACN procedure manual for high-acuity, progressive, and critical care. Seventh edition. ed. Elsevier;2017:xxiv, pp880-884.
68. Xiao HY, Wang YX, Xu TD, et al. Evaluation and treatment of altered mental status patients in the emergency department: Life in the fast lane. World J Emerg Med.2012;3(4):270-7. doi:10.5847/wjem.j.issn.1920-8642.2012.04.006
Clinical Poster Presentations Cited



